 Objective
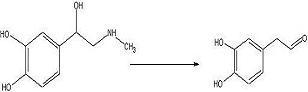
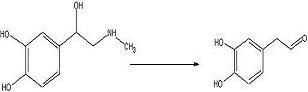
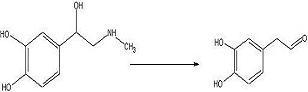
ObjectiveTo convert
adrenaline to a
catechol aldehyde using acid catalysis. How this fits into the synthesis of anti-malarials is described
here.
ProcedureAdrenaline (112 mg, 0.665 mmol) was added to a 50mL round bottom flask with concentrated sulfuric acid (15mL) and benzene (15mL) and water (2mL). The solution was refluxed for 24 hours under nitrogen.
ResultsThe bottom layer (sulfuric layer) turned from yellow to brown to black faster than
Exp 008. Benzene layer remained colorless.
Spotting the benzene layer showed some UV activity however in 2:1 methylene chloride hexanes there seemed to be a very small movement.
The benzene layer at first gradually disappeared however this was fixed after witnessing the reflux condenser
here when taking the first sample.
Start of reflux,
sample 23hrs of reflux,
not seen in other refluxes.
DiscussionIf the aldehyde formed in the benzene layer, which the slight movement on the TLC plate would suggest, in this
before and
after shot ,then the reaction is working. However the concentration is low and the movement can not be seen well. Any amines, including the
adrenaline starting material, should remain in the acidic aqueous phase. The TLC plates were stained with Ninhydrin and it shows that there are no amines in the benzene layer. The samples that were spotted on the TLC plates had very little benzene in them for some reason. They are at the beginning of the reaction so it is assumed that the aldehyde does not form that rapidly. The problem with keeping the benzene layer was fixed by taping the condenser and increasing the
nitrogen pressure.
ConclusionThe desired
aldehyde may have been produced but there is still too little of it. Next try will be an increase in adrenaline of about 3 times this concentration.
Log2006-05-04
13:30. Added all components to 50mL round bottom flask
13:55. Took sample t=0 and turned heat on
14:28. Reflux starts
15:28. Sample t=1hr taken
16:28. Sample t=2hr taken
21:10. Sample t=6.5hr taken, benzene layer depleting, 15mL more benzene added
2006-05-05
9:45. Sample 19hr taken
12:00. Sample 21.5hrs taken
13:40. Sample 23 hours taken
14:30. Sample 24 hours taken, heat turned off, solution capped
2006-05-09
TLC done in 1:1 methylene chloride - no change
TLC done in 2:1 methylene chloride - some movement
2006-05-11
Added 20mL of DI water to round botttom flask and put into 125mL separation funnel
Separated out the acid layer and the benzene layer
Added 10mL more water to acid layer and put back into separation funnel
Added 20mL of benzene, separated and added benzene layers
Put acid layer back into separation funnel and added 30mL benzene
Separated, added benzene layers together and set acid layer aside
Rotovaped the combined benzene layers in 100mL round bottome flask ending with some acid layer still present because Magnesium sulfate was not added.
Took round bottome and added benzene approx. 80mL and 10mL of water, separated then added magnesium sulfate to benzene layer and filtered
Took round bottom flask with benzene and rotovaped ending with a little benzene still left over with a yellowish color.
2006-05-12
Took round bottom flask that was rotovaped which still had some benzene and put it on a vacuum pump. Before doing so the weight of a 50mL round bottom flask was taken (33.86g), the contents of the 100mL r.b.f. was added along with 5mL of methylene chloride, the weight was taken again (40.35g). The flask was put on the vacuum pump and the methylenechloride and benzene were evaporated. The final weight was also taken (33.87g) giving an approximate 10mg of product. A Chromatotron will be done.
2006-05-15
A Chromatotron was performed under nitrogen gas. 10mL of methylene chloride was added to the vacuumed sample and spotted first on a TLC plate and then added to the Chromatotron. A TLC plate was done in pure methylene chloride prior to the chromatotron and the same spot ran quickly suggesting that the same compound is present. The solvent used was 2:1 methylene chloride with hexanes. The bands formed and moved quickly under the force of the moving plate.
Before first fraction After first fraction