Exp 010
Objective:
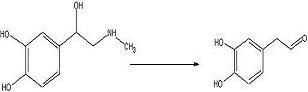
To convert adrenaline to a catechol aldehyde using acid catalysis. How this fits into the synthesis of anti-malarials is described here.
Procedure:
Adrenaline (303 mg, 1.653 mmol) was added to a 100mL round bottom flask that had a spout for a thermometer then added concentrated sulfuric acid (40mL). The solution was heated to an uncertain temperature with a reflux condensor for 14 hours under nitrogen.
Results:
At some point during the night the stir plate stopped stirring and sulfuric vapors escaped through the the condenser and ended up in the oil for the nitrogen bubbling. As seen in the videos the thermometer did not actual reach the liquid layer and the reading it gave was off of fumes from the sulfuric acid.
Evening video Next morning video.
Reaction was stopped, water was added to solution. Tried to separate with methlyenechloride but the acid solution was an emulsion. A quick TLC revealed no UV absorption.
Discussion:
Unsure if sulfuric would still travel up the condenser if more stirring was provided. Benzene layer may be needed for concentrated sulfuric. The temperature was probably to high which would account for the color change in the bubbling oil.
Conclusion
Make sure thermometer is in contact with the solution next time.
Log
2006-5-22
19:50) Added sulfuric acid, and adrenaline to a 100mL r.b.f. with a spout for a thermometer. (Thermometer did not touch solution) Took sample t=0, left over night.
2006-5-23
9:50) Stirrer stopped during the night and sulfuric escaped through the condensor and out the N2 needle. Heat turned off. Sample taken.


0 Comments:
Post a Comment
<< Home