Exp 008
 Objective
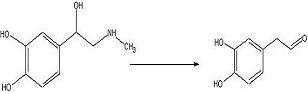
ObjectiveTo convert adrenaline to a catechol aldehyde using acid catalysis. How this fits into the synthesis of anti-malarials is described here.
Procedure
Adrenaline (50.5 mg, 0.283 mmol) was added to a 50mL round bottom flask with 6M sulfuric acid (15mL) and benzene (15mL). The solution was refluxed for 24 hours under nitrogen.
Results
The bottom aqueous layer turned yellowish ,to brown and gradually to black.
The top benzene layer remained colorless.
Spotting the benzene layer at various times on a TLC slide showed no UV activity after evaporation.
The benzene layer gradually disappeared by the end of the 24 hour period.
beginning of reflux video (avi)
End of reflux video (avi)
Discussion
If the aldehyde formed, it would be expected to be extracted into the benzene layer. Any amines, including the adrenaline starting material, should remain in the acidic aqueous phase. Because the aldehyde is aromatic, a spot on a TLC plate should be UV active. Because the benzene layer was not UV active when spotted, it seems that no significant amount of aldehyde was formed.
The loss of the benzene layer may be due to poor reflux condenser-round bottom flask connection.
Conclusion
The desired aldehyde was not produced in refluxing benzene/6M sulfuric acid over 24 hours. A stronger acid concentration might work.
Log
2006-04-24
17:25] First sample taken and heat turned on under N2.
18:05] Reflux starts
19:15] First reflux sample taken 1hr 10mins from start of reflux
20:05] Second reflux sample taken 2 hrs; some yellowish color
2006-04-25
12:20] Third reflux sample taken 18hrs 15mins; light brown color; benzene layer has decreased
15:20] Fourth sample taken 21hrs 15mins; dark color and diminishing benzene layer
16:20] Fifth reflux sample taken 22 hrs 15mins; benzene layer is gone
17:25] Sixth reflux sample taken 23hrs from start of reflux; heat turned off reflux stopped.
2006-04-27
TLC were done in 1:2 methylenechloride and hexanes. There was no UV activity.


