Exp 011
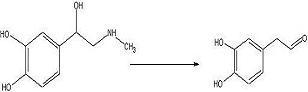
Objective:
To convert adrenaline to a catechol aldehyde using acid catalysis. How this fits into the synthesis of anti-malarials is described here.
Procedure:
Adrenaline (302 mg, 1.653 mmol) was added to a 100mL round bottom flask with concentrated sulfuric acid (40mL) and a benzene layer (27mL+10mL). The solution was refluxed 24 hours under nitrogen.
Results:
The start of the reaction clearly showed two layers here.
After 40minutes there seemed to be only one phase. This video may be deceiving because there is a reflection on the round bottom flask giving the appearance of 2 layers.
A TLC of a small benzene/water extraction (benzene layer) after 24 hours showed little signs of anything UV active.
Discussion:
We would expect the catechol aldehyde to partition in the organic phase of a benzene/aqueous sulfuric acid mixture and be UV active when spotted on a TLC plate, but did not observe this even after 24 hours of reflux.
Here is paper on an experiment that used silica sulfuric acid and benzene to make benzene sulfonic acid. Chances are good that is what happened. Possibly the sulfuric acid concentration should be reduced or the concentration of adrenaline which can act like a soap between the benzene and acid layer.
Conclusion:
Refluxing adrenaline in a mixture of benzene/sulfuric acid for 24 h did not produce the desired aldehyde.
Log:
2006-5-24
1700hours The bubbler oil is brown from Exp 010 but the reaction starts and heat has been turned on. Took sample.
1740hours Where is the benzene layer? The volume is the same and there is only a small benzene layer. Took sample.
19:40)before leaving added 10mL more benzene. Sample taken.
2006-5-25
9:40) Volume of solution was the same. No sample taken.
17:20) Heat turned off. Flask and solution were allowed to cool under nitrogen while water continued through the condenser.
18:00) Turned off water and nitrogen and put parafilm over flask.


0 Comments:
Post a Comment
<< Home