Exp 013
Objective:
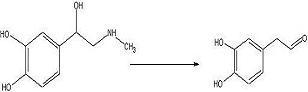
To convert adrenaline to a catechol aldehyde using acid catalysis. How this fits into the synthesis of anti-malarials is described here.
Procedure:
Adrenaline (215 mg, 1.173 mmol) was added to a 100mL round bottom flask with glacial acetic acid (40mL) and water (4mL). The solution was refluxed 24 hours under nitrogen.
Results:
Took round bottome flask of sample 13E, and evaporated the 40mL of acetic down to 10mL. 2hr video 10hr video 24hr video
TLC results of what remained from vacuum pump show some movement in 1:1 methylene chloride methanol. Also in pure methanol.
Discussion:
Analyzing peaks and waiting for spinning to be fixed. Concentration of any product may have been too little however the number of scans was also insufficient. Next reaction will be more concentrated.
Log:
2006-5-30
9:30) sample taken (13A), solution yellow
10:57) solution boiling with slight reflux, heat raised, solution still yellow, sample taken(13B)
11:20) reflux is insufficient, heat turned up to produce better reflux
14:56) sample taken(13C), reflux is better but slow, no changes in color
20:10) sample taken(13D), solution is still yellow, reflux is decent
2006-5-31
8:15) solution did not change over night, sample taken(13E) reflux was still decent
2006-6-2
Took C13 on Varian 300MHz NMR. Concentration wa approximately 20mg(combination of unreacted adrenaline and any product that formed)/1mL acetic acid
2006-6-9
15:00)Vacuumed off the remaining acetic acid. A dark polymer like solid remained.
16:00)Scrapped a little off and dissolved it in 1-1.5mL methanol.
16:40)TLC in 1:1 methylene chloride hexanes showed nothing.
16:50)TLC Pure methylene chloride showed nothing.
17:00) 1:1 methylene chloride methanol produced some movement.
17:10) Pure methanol produced about the same result as the 1:1 methylene chloride in methanol.
0 Comments:
Post a Comment
<< Home